Cell & Gene Therapy
Integrated solutions for
Advanced Therapies
Medicinal Products
Integrated healthcare order-2-cash
logistics is our home turf
Integrated vein-to-vein logistics is in this fast-growing environment of Cell & Gene Therapies one of the differentiating keys to success. The setup is as individual as every patient’s therapy in terms of transportation time, storage requirements, temperature control and handling sensitivity. Beside this, all involved stakeholders must ensure that everybody has the right information as the right time for their activities. Based on our clients’ individual requirements, we design, implement and operate an efficient and patient-centric supply chain with the right IT platform that integrates processes and stakeholders.
Based on our many years of experience and knowledge, we now support your Cell & Gene Therapy – from patient registration to reimbursement – out of one hand.

We provide innovative integrated solutions for patient individual supply chains with digital applications and operational excellence that manage complexity and provide transparency to support key stakeholder.
Thorsten Winkelmann President Healthcare
Integrated services for manufacturers of Cell & Gene Therapies
Manufacturers need to be supported along the entire patient journey
Cell & Gene therapies are highly complex processes with a lot of bureaucratic paperwork and many stakeholders that must be managed and streamlined. The supply chain is completely different compared to traditional pharma logistics. Therefore, new supply chain strategies must be implemented that give the rights answers to these changes and challenges:
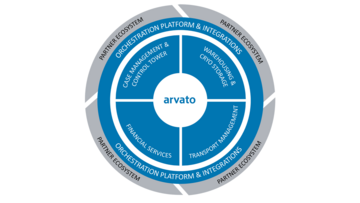
Our end-to-end service solution
This entire process - from order to reimbursement - needs to be designed as a “fully integrated service model”. Healthcare manufactures will only be able to scale commercialization of their Cell & Gene therapy when they reduce process complexity, increase transparency and care for quality & security.
We are the right partner for the healthcare industry and manufacturers of advanced & personalized therapies: Our true end-2-end solution provides the right services, processes and orchestration platform that enable successful therapy delivery to increase quality of life.
Cell & Gene Orchestration Plattform
We take care that every stakeholder gets the right information at the right time to delivery the right therapy to the right patient!
The Cell & Gene Orchestration Platform is an enabling differentiator to provide ATMP to your patients successfully.
Advanced and Personalized Treatments like Cell & Gene Therapies demand safe and seamless collaboration across the supply chain to uninterruptedly safeguard the Chain-of-Identity (CoI) and Chain-of-Custody (COC). The platform provides transparency across the full treatment cycle to all parties so that a smooth delivery of the therapy is ensured.
- Real-time supply chain orchestration along all involved organizations and roles
- Customizable, flexible workflows: From registration to reimbursement
- Scheduling and capacity planning for the entire treatment journey
- Carrier booking together with track & trace visibility
- Adaptable label printing engine in accordance with ISBT128
- FDA 21 part 11 compliant digital signatures
- Compliant audit trails proofing CoI & CoC, optionally based on immutable blockchain technology
Case management
We manage complexity and provide the right transparency!
Successful therapy delivery needs operational case managers who oversee, coordinate, and if needed, troubleshoot logistics to ensure that starting material or manufactured products arrive on time, every time.
Our operational case managers support our Clients and key stakeholders by navigating the complexities of cell therapy delivery:
- 24/7 availability and able to support in local language
- Align all stakeholders to ensure seamless therapy delivery
- Coordinating and scheduling logistics: from collection over manufacturing to infusion
- Tracking and verifying CoI & CoC
- Troubleshooting and providing solutions to unexpected challenges
Warehousing and Cryo storage
We take care of your cold-chain management!
Delivery of cell and gene therapies requires the ability to hold products in readiness whilst logistical, regulatory and potency considerations are dealt with and recorded. Arvato’s site network is equipped with state-of-art infrastructure and supports your cold-chain requirements.
- 200,000 m² dedicated warehouse space at 15 sites
- Temperature Controlled Areas (+15°C to +25°C, +2°C to +8°C, -20°C, -80°C and -196°C (LN2))
- Sites in Germany, France, the Netherlands, UK, Ireland, Switzerland, Spain, Portugal, Italy, Poland and USA
- GMP + GDP certifications
- ISO 9001:2015 & ISO 14001:2015
- QP-Onsite
- Unified and integrated QA system
Transport management
We ensure that your therapy arrives on the right time, at the right place for the right patient!
The ultimate success of cell and gene therapy products relies on the seamless delivery to patients. Many gene therapy products and intermediates need to be stabilized by either cryogenic perseveration or refrigeration during storage and transport with limited or no allowable temperature excursion. Transport management services are be available for each country and required delivery service using validated carriers.
- Customized transport solutions for highly sensitive and urgent products
- Individual cold-chain packaging solutions (e.g. special dry shipper for Cell & Gene transports)
- Transport of actively or passively cooled products from ambient to cryogenic (+25°C to -196°C)
- Track & Trace (e.g. GPS, temperature, humidity, Pressure, light, orientation, shock)
- Expert knowledge: Transportation, customs, legal, compliance
- Network with >150 carriers
Financial services
We take care of your financial flows to bring your therapy successfully to your patient!
Even cell and gene therapies request to manage financial flows. The financial services for advanced therapies consists of services such as creation and dispatch of invoices, accounts-receivable (AR) management, dunning, factoring. All services are conducted in full compliance of SOX requirements.
- Financial services on behalf of the manufacturer
- Activities will be performed in the “look and feel” and in accordance with the corporate design of the client.
- Document in local language and currency and respecting the applicable VAT
- Professional customer care service: “one face to the customer” by dedicated financial service team
- Process-oriented and integrated consulting: all components of financial management from one source
Our partners
Any failure within the supply chain of an ATMP can have the same impact on a patient’s life as a failure within the manufacturing process. Therefore, it is essential to have the right partners on board that support and ensure the successfully delivery of very critical shipments – whether in a clinical or in commercial environment.
We have a network of specialized partners, who meet our high quality and process standards. The dedicated alliance ensures that we provide the best possible service to our clients in over 125 countries.

Membership
ISCT is the global leader focused on translational aspects of developing cell-based therapeutics, advancing scientific research into innovative treatments for patients. Through long term strategic relationships with global regulatory agencies, academic institutions and industry partners, ISCT leads the advancement of research into clinical adoption and standard of care.